The next step in NeuroRehabilitation
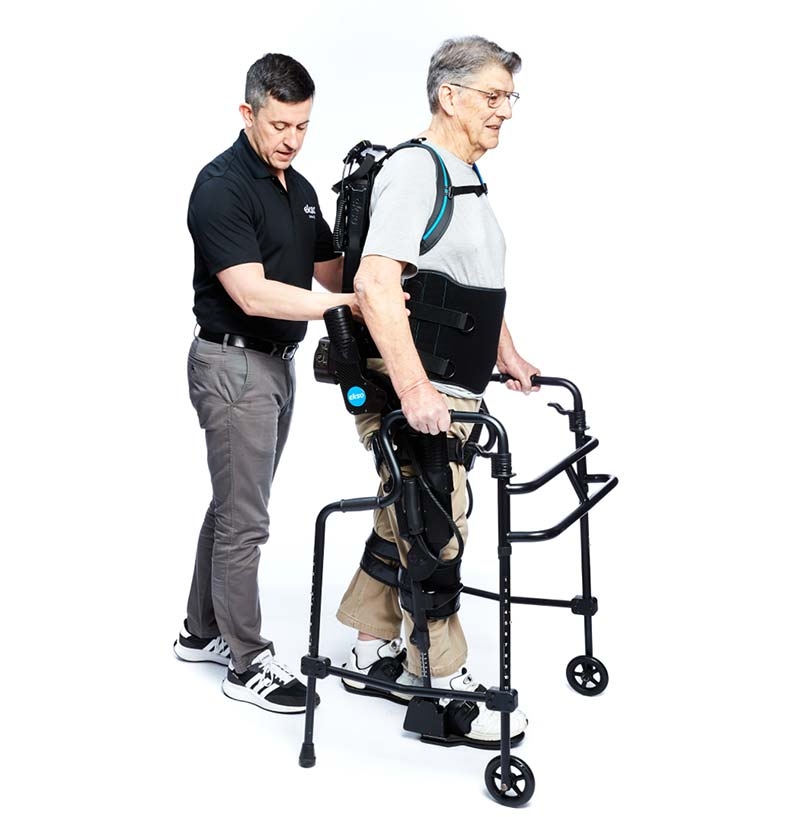
EksoNR is a robotic exoskeleton specifically designed to be used in a rehabilitation setting to progress neurorehab patients so they can walk out of the device and back into their communities. As the first exoskeleton FDA-cleared for acquired brain injury, stroke, multiple sclerosis (MS) and spinal cord injury, EksoNR offers the industry’s most natural gait, re-teaching the brain and muscles how to properly walk again.

Announcing new, intuitive GaitCoach Software for EksoNR!
Allow us to introduce GaitCoach, our newest software for EksoNR. We listened to your feedback and created tools that speak your language, making it easy to learn, easy to use, and easy to progress your patients. Just stand, start walking and let GaitCoach monitor everything your patient does. Any time GaitCoach spots a component of your patient’s gait or posture that needs work, it notifies you. GaitCoach is here to make your job as a therapist easier. It monitors your patient and offers additional feedback and guidance, so you can rely on your clinical expertise to decide how to best treat your patient. If EksoNR is your extra set of hands, then GaitCoach is your extra set of clinical eyes.
Learn More
Features
GAITCOACH SOFTWARE
GaitCoach analyzes components of walking and notifies the therapist what to focus on to improve gait quality.
CLINICIAN CONTROL
Modify swing and stance assistance levels in real time based on patient goals and GaitCoach provided feedback.
POSTURE SUPPORT
EksoNR helps patients bear only their own weight while assisting with postural alignment through the trunk so you can treat patients through the continuum of care.
DATA CAPTURE
View session-specific data including walking time, distance, and speed, securely saved to a cloud-based dashboard for easy analytics.
PATIENT ENGAGEMENT
Patients actively participate to correct gait deviations based on visual and auditory feedback provided by GaitCoach.
PROGRESSIVE GAIT TRAINING
Intelligent software continuously monitors the walking pattern and guides the therapist to progress the patient to maximize intensity.
MORE THAN WALKING
EksoNR includes pregait activities to help patients balance, weight shift, squat, and step in place before walking.
Looking to learn more about EksoNR and it’s technology? Download our technical brochure for all the answers.
Download PDFSimplified robotics that speaks your language
EksoNR is the only exoskeleton FDA-cleared for ABI and MS, in addition to stroke and SCI. It was designed to be a rehab tool for physical therapists to challenge their patients, requiring active participation known to drive brain plasticity. We listened to hundreds of Ekso-trained therapists and simplified EksoNR to be more intuitive, easier to use, and faster to train while still maintaining broad customization for each patient. Our new software, GaitCoach, provides guidance throughout each session, identifying gait deviations and providing tailored feedback to improve on specific components of gait.

Lead the way in NeuroRehab
EksoNR promotes upright posture and natural gait, so therapists can focus on treatment. The high, rigid back and GaitCoach guided progressive modes make EksoNR the ideal solution for patients just beginning to walk or those working on improving quality gait mechanics. EksoNR with GaitCoach is the only mobile exoskeleton offering guidance to the therapist regarding postural and gait deficits with real time feedback on how to improve and progress the patient. GaitCoach+ is an upgrade that builds off what GaitCoach software provides, giving you more options to further customize your sessions.
GaitCoach+ is intended for experienced EksoNR users who want to expand what they can do with their patients. Some of the additional features and capabilities of GaitCoach+ include step in place, squats at varying depths, separation of swing assistance and stance support, the ability to choose different options for the right and left leg, adding resistance, and additional settings to optimize patient outcomes. EksoNR with GaitCoach+ adds additional opportunities for highly Ekso-experienced therapists to address a further range of goals and get even more out of patient sessions.

INCREASE DOSAGE
As compared to conventional therapy, patients take more steps and walk further distances when their plan of care includes EksoNR.
GAIT SPEED
Including EksoNR gait training during rehabilitation improves patient gait speed outside of the device at discharge compared to admission.

WALKING DISTANCE
Research shows that EksoNR gait training improves walking distance outside of the device for people with stroke and motor incomplete SCI at discharge, when compared to patients rehabilitated without Ekso.
Backed by data from over 40
industry-leading research partners
EksoNR was developed by clinicians for clinicians. With more than 2,000 patients participating in over 185 clinical trials, EksoNR is the most researched mobile exoskeleton on the market. Learn how training with EksoNR can improve the limitations in walking ability caused by neurological disorders, along with their physiological and psychological consequences, thus improving patients' quality of life.
See our evidenceEksoNR FAQs
Over 500 devices are in 30+ countries worldwide. More than 30% of centers with Ekso have more than one device.
Contact us to get connected with a Regional Manager to discuss your program and whether EksoNR would be a good fit at your facility.
EksoNR is the only exoskeleton designed specifically by clinicians for clinicians and features software incorporating early mobility tools and independent walking support. EksoNR is also the only exoskeleton FDA cleared for use with acquired brain injury and multiple sclerosis (MS).
GaitCoach analyzes the patient’s walking and provides guidance to the therapist regarding specific aspects of gait. The choices in the software are greatly simplified, making the training and use of the device easier. If you are interested in upgrading to GaitCoach software, please contact us.
EksoNR fits patients under 220 pounds and between 5′ and 6’4″.
EksoNR comes with two sets of rechargeable lithium ion batteries that allow for continuous use.
Ekso Bionics exoskeletons are only available for patients from 5′ to 6′ 4″ and is FDA cleared for use for those who are 18 years old and over. Please contact Customer Relations for more information.
No, EksoNR is only available in certified rehabilitation centers. Find a center near you with our interactive map. If you are interested in a personal exoskeleton for home and community use, check out our Ekso Indego Personal for more information.
Step in place, squats, separation of swing and stance assistance and support, the ability to choose different options for right vs left leg adding resistance, and additional customizations. Contact us to learn more.
