Indications for Use
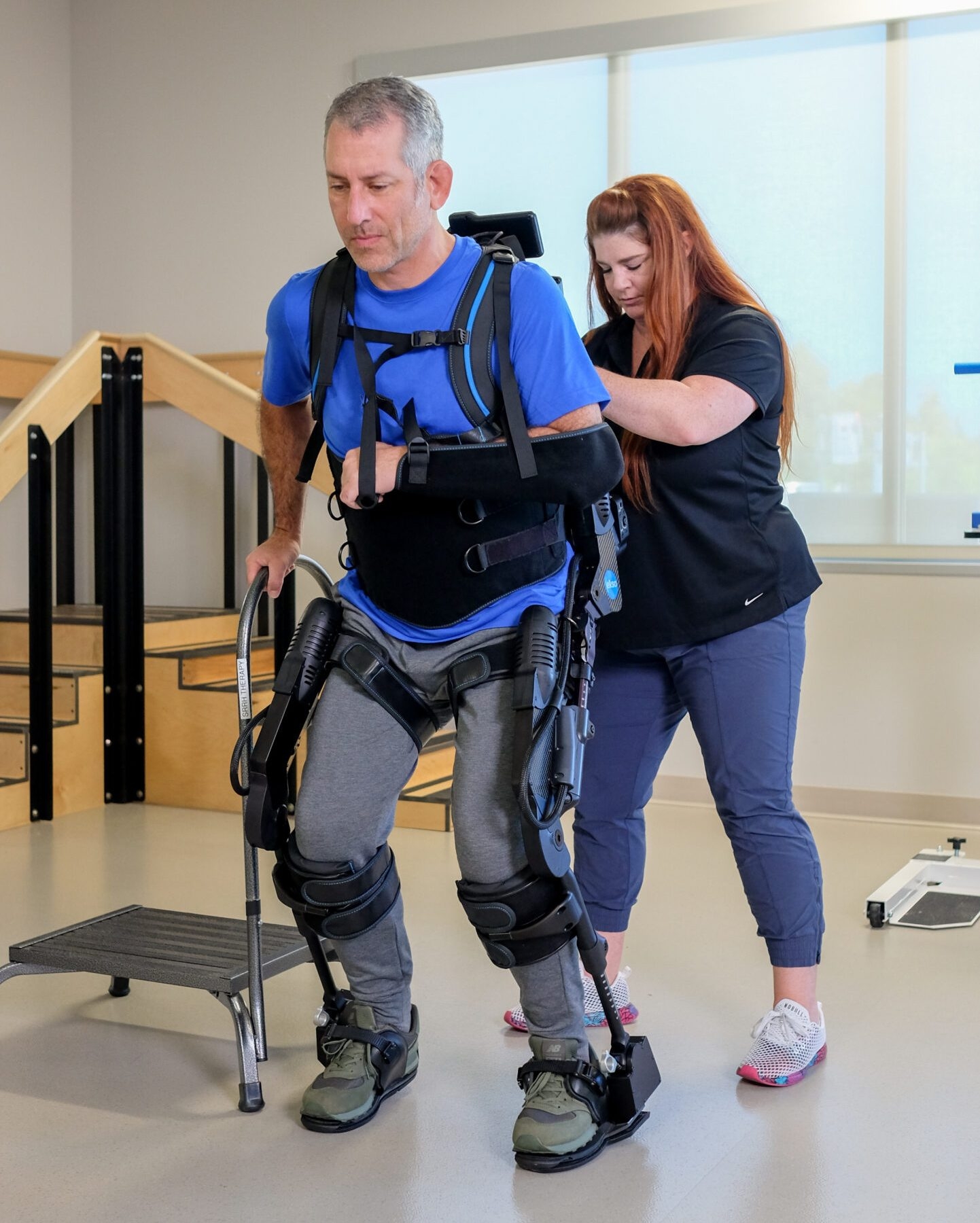
Indications for Use – EksoNR (USA)
EksoNR is intended to perform ambulatory functions in rehabilitation institutions under the supervision of a trained physical therapist for the following populations:
- Individuals with multiple sclerosis (upper extremity motor function of at least 4/5 in at least one arm)
- Individuals with acquired brain injury, including traumatic brain injury and stroke (upper extremity motor functions of at least 4/5 in at least one arm).
- Individuals with spinal cord injuries at levels T4 to L5 (upper extremity motor function of at least 4/5 in both arms)
- Individuals with spinal cord injuries at levels of C7 to T3 (ASIA D with upper extremity motor function of at least 4/5 in both arms)
The therapist must complete a training program prior to the use of the device. The devices are not intended for sports or stair climbing.
Source: FDA Part 21 CFR
Indications for Use – EksoNR (EU)
- EksoNR is intended for use as a gait training device to improve walking function and independence in patients with a neurological or muscular injury, illness, or weakness.
- EksoNR is designed to be used in a controlled clinical or non-clinical setting under supervision of Ekso Certified Physical Therapist (or equivalent medical professional) and operated by a Trained Spotter.
- EksoNR is a device intended to help facilitate the restoration or improvement of ambulation for its pilots. The device is intended to serve multiple pilots over the duration of its life.
- EksoNR is used to manipulate the legs of a patient and move them through the normal walking gait, from the sit-to-stand position and from standing back to sitting.
Source: Intended Use – Council Directive 93/42/EEC, Annex II
Indications for Use – Ekso Indego
Personal & Ekso Indego Therapy
The Ekso Indego® orthotically fits to the lower limbs and the trunk; the device is intended to enable individuals with spinal cord injury at levels T3 to L5 to perform ambulatory functions with supervision of a specially trained companion in accordance with the user assessment and training certification program. The device is also intended to enable individuals with spinal cord injury at levels C7 to L5 to perform ambulatory functions in rehabilitation institutions in accordance with the user assessment and training certification program. Finally, the Ekso Indego® is also intended to enable individuals with hemiplegia (with motor function of 4/5 in at least one upper extremity) due to cerebrovascular accident (CVA) to perform ambulatory functions in rehabilitation institutions in accordance with the user assessment and training certification program. The Ekso Indego is not intended for sports or stair climbing.
Source: FDA
